The Chapman group investigates the viral-host interactions of the gene therapy vector, adeno-associated virus (AAV), using biophysical and molecular approaches. Cryo-Electron Microscopy (EM) is now prominent in our characterization of AAV complexes with cellular receptors and antibodies.
Structural Virology – Host Interactions:
The US Food & Drug Administration has now approved four gene replacement therapies: Luxturna™ (2017), a treatment for congenital blindness, Zolgenesma™ (2019) for spinal muscular atrophy (SMA), a debilitating and fatal genetic disorder for which thousands of infants have now been treated, and then, in the last year, treatments for both hemophilia A and B. These, and other treatments in development, use recombinant rAAV vectors to deliver DNA to afflicted cells. Our structure-function analyses provide a fundamental understanding of the atomic interactions key to cell entry, trafficking, and immune neutralization. Our foundations are needed for the engineering of gene therapy vectors that are efficient and specific enough to treat an array of genetic diseases at safe dose levels.
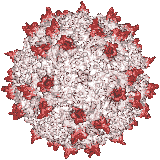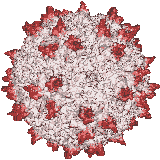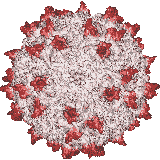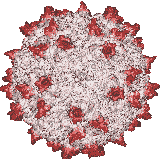 Our X-ray crystallographic structure of AAV-2 in 2002 (right) opened the door to the structural virology of AAV. Glycans had been implicated as cellular receptors for different AAV serotypes, heparan sulfate proteoglycan (HSPG) for AAV-2. However, our structures of complexes, glycan binding studies and mutational analyses showed that the specificity was not like that expected of classical receptors. Looking for alternatives, our attention turned to co-receptors implicated in the literature, but we were unable to get evidence of direct interactions. Thus, we embarked on a genome-wide screen for cellular factors essential in AAV transduction. Collaborating on a gene trap screen with Jan Carette (Stanford), in 2016 we identified a hitherto uncharacterized membrane protein, KIAA0319L. It is now called AAV Receptor or AAVR and is the most important of several key host proteins in the entry of AAV and its trafficking towards the nucleus. We have since identified the key AAVR domains, expressed them, and determined structures of complexes with several AAV serotypes by cryo-EM at resolutions of up to 2.4 Å. These are sufficient to reveal amino acid interactions at the binding interface.
Our X-ray crystallographic structure of AAV-2 in 2002 (right) opened the door to the structural virology of AAV. Glycans had been implicated as cellular receptors for different AAV serotypes, heparan sulfate proteoglycan (HSPG) for AAV-2. However, our structures of complexes, glycan binding studies and mutational analyses showed that the specificity was not like that expected of classical receptors. Looking for alternatives, our attention turned to co-receptors implicated in the literature, but we were unable to get evidence of direct interactions. Thus, we embarked on a genome-wide screen for cellular factors essential in AAV transduction. Collaborating on a gene trap screen with Jan Carette (Stanford), in 2016 we identified a hitherto uncharacterized membrane protein, KIAA0319L. It is now called AAV Receptor or AAVR and is the most important of several key host proteins in the entry of AAV and its trafficking towards the nucleus. We have since identified the key AAVR domains, expressed them, and determined structures of complexes with several AAV serotypes by cryo-EM at resolutions of up to 2.4 Å. These are sufficient to reveal amino acid interactions at the binding interface.
There have been two surprises. AAVs exhibit two distinct modes of receptor-binding. AAV5-like viruses bind exclusively to the first of five polycystein kidney disease (PKD) domains, whereas other clades of AAV bind primarily to PKD2 at a different location on the virus surface. Secondly, our EM structure of an antibody complex shows that monoclonal mAb A20 conflicts with AAVR, surprising with the prior view that neutralization by A20 and other antibodies was post-entry. Similar results from AAV-5 force us to re-evaluate antibody neutralization mechanisms. It seems that the consensus on neutralization had been built on the incorrect conclusion that glycans were the entry receptors. Our work has established that the glycans are non-specific attachment factors that are bound in a distinct step prior to AAVR-mediated endosomal entry. A full understanding of viral entry and of surface epitopes bound by neutralizing antibodies are prerequisites for the rational engineering of AAV capsids to be designed for more efficient targeting of specific cells and immune evasion. Thus, we continue our structural investigations, characterizing interactions with AAVR and other host proteins on AAV’s path from the cell surface to the nucleus.
Electron Microscopy
EM has become the method of choice for virus structural biology. The Chapman and Stagg groups have been on the forefront, applying emerging technology in achieving new milestones. Our glycan complexes were the first to reveal functional groups of small ligands bound to a target, and our structure of AAV-DJ at 1.56 Å resolution has only recently been overtaken by apoferritin as the highest resolution biomolecular structure. Michael Chapman has been committed to building the infrastructure needed by structural biologists, as one of the founding PIs of one of three NIH-supported National Cryo-EM Centers, and in overseeing a new center at the University of Missouri. The Chapman group is active in the development of computational methods for the robust analysis of cryo-EM maps. This is both essential in harnessing EM for advances in AAV biology, and a research project of service to the broader structural biology community.
Computational Methods Development
At the heart of our methods development is a mathematical approach to calculating the expected map values from the contributions of neighboring atoms. It is unique in rigorously accounting for the experimental resolution. This allows accurate comparison of atomic models with EM maps, supporting sensitive calibration of EM magnification and gain in EM reconstructions and the elimination of artifacts in difference maps that are used to analyze ligand-binding and conformational change. Such artifacts are a challenge for the majority of EM studies that continue to be at resolutions below 3.5 Å, where validation through recognition of chemical features become possible. Like other methods, our atomic refinement jointly optimizes fit to the map and model stereochemistry, achieving precision that is 3 to 5-fold improved over nominal resolution. Our rigorous accounting for resolution provides unique advantages: high precision, B-factor refinement, support for sharpened maps and for refinement tested over wide resolution regimes from 1.5 to 25 Å.
On-going work includes refactoring of prototype methods into a user-friendly package, and development of low-order model parameterizations to reduce the over-fitting that is prevalent in low resolution EM refinements. Our approach to eliminating over-fitting is based on refinement of torsion angles rather than atomic coordinates directly, and our earlier discovery, from NMR analysis of protein dynamics, that conformational changes in nature are predominantly parsimonious. Thus, we can further reduce over-fitting by adding a parsimony restraint. Our goal is a new approach to the refinement of atomic models that is benchmarked for robust improvement of accuracy from the highest near-2Å EM resolutions to the nanometer resolutions of electron tomography used to map domain-level conformational change in large complexes.
